‘Swimming pool for bacteria’: There could be life on Mars today - new study
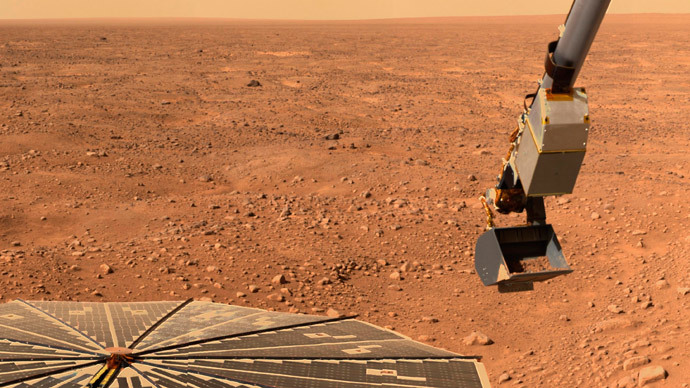
Water, near and on the surface of Mars, melts rapidly enough when combined with salt to allow bacterial life to flourish, despite the freezing temperatures on the planet, a new study from the University of Michigan claims.
The discovery came after NASA expert at the time, Nilton Renno, noticed droplets of what seemed to be water on the robotic arm of the Phoenix spacecraft when it landed on the Red Planet in 2008. Back then, the “droplets” prompted a huge debate, with scientists arguing if they were really ice or actually water – crucial to allowing life to grow – and if the latter, how it melted in temperatures that vary from -120 C to -21 C.
Since taking up a professorship at Michigan in 2012, Renno has been able to construct a “replica” of the exact conditions that occurred during the landing, to prove his belief that water could melt – building a cylindrical chamber with air pressure just 1 percent that of the Earth, as on Mars.
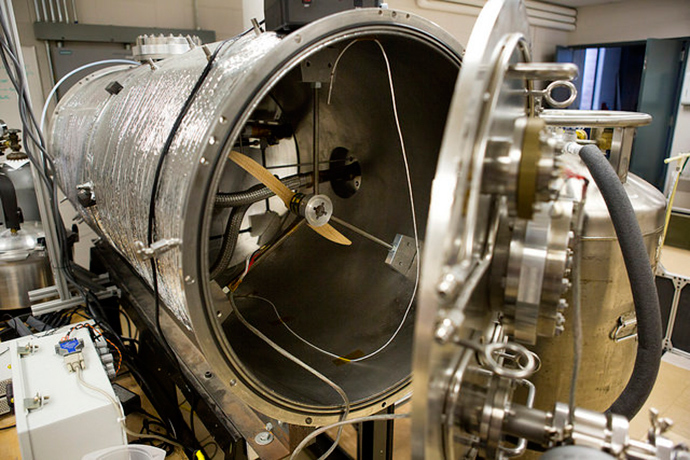
Renno’s experiments have shown that the secret ingredient is salt, or rather Martian salt – calcium perchlorate, which is composed of calcium, chlorine and oxygen.
When it was placed directly next to the ice – ubiquitous on Mars – it can melt it within minutes, producing water.
"For me, the most exciting thing is that I can now understand how the droplets formed on the Phoenix leg," said a delighted Renno, whose initial theories about the presence of salt have been vindicated when more of it was found by both the Phoenix, and the subsequent Curiosity missions.
“Based on the results of our live experiment, we expect soft
ice to liquefy a few days per year, perhaps a few hours per day,
almost anywhere on Mars,” said Renno, noting that the
process was more likely at the polar extremities.
Although the Phoenix artificially aided the process by mixing up
the ice and salt with its arm, water reservoirs the size of
droplets could be produced naturally with no problem.
“This is a small amount of water, but for bacteria, this can be a swimming pool, a huge amount of water. So this is enough to create conditions for Mars to be habitable today,” said Renno.
“Perhaps there is some bacterial life that could replicate and multiply.”
While others in the team are more cautious about whether the conditions for bacterial life actually translate into bacterial life being there, the experiment helps to contribute to the heated debate on whether planets outside our system also frequently contain water. If this is the case, our chances of finding extraterrestrial life increase measurably.

"Mars is the planet in our solar system that is most similar to Earth. Studies suggest that Mars used to be even more Earth-like in the past, with flowing water on the surface. By studying the formation of liquid water on Mars we can learn about possibilities of life outside Earth and look for resources for future missions," said Erik Fischer, doctoral student in the Department of Atmospheric, Oceanic and Space Sciences (AOSS) and first author of the new paper, which has been accepted for publication by the journal Geophysical Research Letters.














