Future cell phones to recharge from air after rain?

MIT scientists have discovered a way to get an electric current out of water droplets. Enough power to charge mobile phones can be drawn from water condensing and jumping from a super-hydrophobic surface.
Researchers from Massachusetts Institute of Technology (MIT) have come up with a simple device that – not without some improvements – could power electronic devices with ‘green’ energy.
The study by postdoctoral scholar Nenad Miljkovic, Associate Professor of Mechanical Engineering Evelyn Wang, and two others was published online in the journal Applied Physics Letters.
The new technology functions on a basic physical principle: water condenses and jumps away from certain kinds of surfaces, called hydrophobic as they repel water, and is attracted to other ones, hydrophilic. The beauty of this subtle process is emphasized by its name: the Lotus effect.

The generator, which is able to get electricity out of thin air, is comprised of two arrays of metal plates, with water droplets moving from hydrophobic to hydrophilic surfaces. Jumping like kangaroos that bring charge from one plate to another, water droplets create charge difference that can be harnessed to provide power.
Although the initial tests involved copper plates, any conductive metal would do, including cheaper aluminum, said Milijkovic.
Furthermore, the scientists claim, the device has a side benefit: the system could also produce clean drinking water.
“Water will condense out from the atmosphere, it happens naturally,” Milijkovic told MIT. “The atmosphere is a huge source of power, and all you need is a temperature difference between the air and the device.”
However, some constraints are inevitable. A cave or river would be ideal, as the device requires a humid environment, and a source of temperatures should be colder than the surrounding air.
Though the amount of power produced in initial testing was really small – just 15 picowatts, or trillionths of a watt, per square centimeter of metal plate – scientists are quite positive about the outcome. Miljkovic says the process could easily be upgraded to achieve at least 1 microwatt, or millionth of a watt, per square centimeter.
According to calculations, it would take 12 hours to fully charge a mobile phone at such an energy production rate – and one would need a device with proportions of a cube measuring about 50cm per side. This could be a good result for people in remote areas, who may have few alternatives.
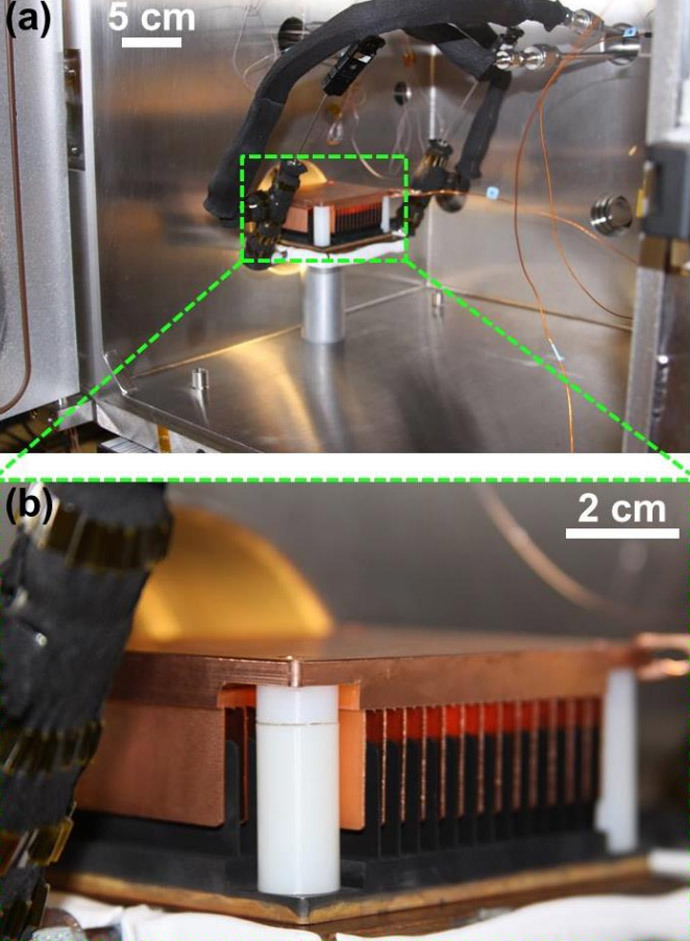
The latest finding is based on another MIT discovery. Back in 2013, researchers found out that when water droplets spontaneously jump away from superhydrophobic surfaces during condensation, they can gain electric charge in the process. Initially, the team tried to develop an improved heat-transfer surface that could work as a condenser, but over the course of the year they turned from power plants to much more compact devices.
Chuanhua Duan, an assistant professor of mechanical engineering at Boston University who was not involved in this research, said, “Getting power from a condensation process is definitely a novel idea, as condensation is mainly used for thermal management.”














