US spends most on this drug… and no one knows how it works
Americans spent over $7.5 billion on one drug over a one-year period ‒ more than any other medication. And yet no one knows how the powerful pill works. But that doesn’t keep Big Pharma from marketing it for a multitude of disorders.
“Quick: what’s the top-selling drug in the United States?” the Daily Beast asked. “Prozac? Viagra? Maybe something for heart disease?”
The answer is actually Abilify (aripiprazole), a powerful antipsychotic used to treat schizophrenia and bipolar disorder, among other major psychiatric conditions.
Americans spent over $7.5 billion on the drug between October 2013 and September 2014, with nearly 8.8 million prescriptions filled per month in that same time period, Medscape Medical News reported. The sales of aripiprazole were more than all other major antidepressants combined.
Bristol-Meyers Squibb (BMS) initially developed Abilify to treat schizophrenia ‒ a disease affecting only one percent of Americans. A few years later, it was approved for bipolar disorder, an illness that an additional 1.5 percent of Americans battle. But then the Food and Drug Administration (FDA), which approves and regulates all medications in the US, allowed the drug to be used in conjunction with other medications to treat major depressive disorder, MedShadow Foundation’s Suzanne B. Robotti reported.
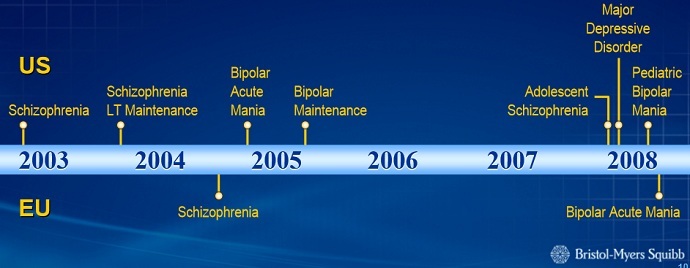
Depression affects 16 million people in the US, Dr. Candida Fink, an expert in bipolar disease, pointed out to Robotti, adding that it is a significant and lethal disease.
Once the drug was able to be marketed as an add-on for depression, its sales soared. (BMS spends $121 million on promoting Abilify each year, according to MedShadow.) Its advertisements tout that the drug works “like a thermostat to restore balance,” and that “[when] activity of key brain chemicals is too high, Abilify lowers it…. When activity of key brain chemicals is too low, Abilify raises it.”
The Daily Beast likened the drug to “a bazooka to conventional anti-depressants’ revolver.”
That’s about as close as anyone gets to explaining how the powerful, atypical antipsychotic works.
“Our promotional activities focus on the description of the mechanism of action of aripiprazole as it is written” in the standardized United States Product Insert (USPI), a spokesperson for Otsuka, which produces Abilify with Bristol-Meyers Squibb, told the Daily Beast.
But the USPI doesn’t shed any light on how the drug works. In fact, it says that the “mechanism of action of aripiprazole… is unknown.” It suggests a “proposed” way of working, but does not list any evidence supporting the theory. The FDA also says the way Abilify works is “unknown.”
A page called "How Abilify is thought to work" on the the drug's website notes, "Keep in mind that the exact way ABILIFY works has not been fully determined."
An article in PLoS Medicine, a peer-reviewed journal, called the advertisements “questionable,” asking “whether the complexities of treating bipolar disorder (with its unknown etiology and well-known heterogeneity in response to treatment) are accurately portrayed as a reliable, mechanical thermostat.”
“However,” the authors noted, “consumers are likely to find such advertisements compelling.” The lack of government regulation or outcry from professional associations about Otsuka’s claims, they hypothesized, “significantly contributes to the process of disease mongering.”
“The advertising is everywhere,” Fink told Robotti. “The effects of the advertising are very vivid to me, I see it all the time. I talk to my patients about making the best decision for them and not rely on advertising. But that’s certainly a big part of why Abilify is selling as much as it is ‒ because of the advertising.”
And patients who rely on television ads are the targets of what the PLoS authors describe as disease mongering, which is another way for pharmaceutical companies to grow sales.
“Consumers who view such advertisements are likely to characterize their problems in a manner congruent with industry promotion and to request well-advertised pharmaceuticals as treatment,” they wrote. “At a bare minimum, increased medicalization will result; in some cases, disease mongering may indeed be an appropriate characterization.”
Abilify is also used to treat irritability and symptoms of aggression, mood swings, temper tantrums and self-injury related to autistic disorder in children who are at least 6 years old, Drugs.com noted.
Although aripiprazole is the top grossing medication in the US, it is only the 13th most prescribed. Crestor, a cholesterol-lowering drug, edged out Synthroid, a hypothyroid medication, for the top spot, with almost 22.28 million a month between October and September, compared to 22.56 million per month. But Crestor’s sales were almost $5.8 billion over that one year period, while Synthroid’s totalled less than $1 billion, according to Medscape.