‘A step to reversing death’: New trial hopes to revive brain-dead patients
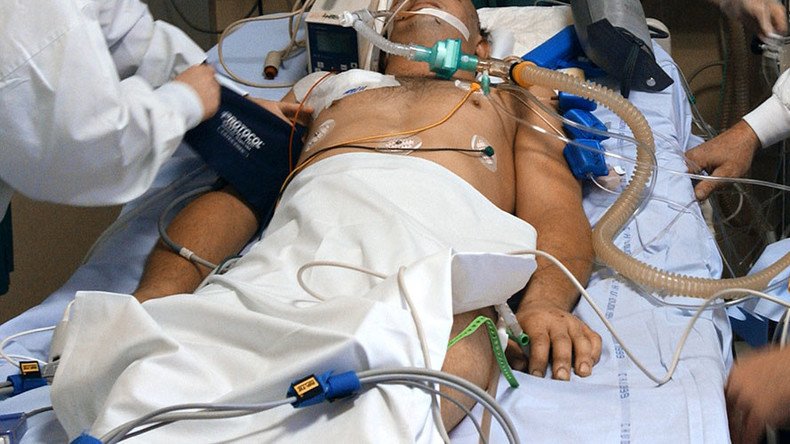
A US biotech company has been given regulatory approval to recruit 20 patients with no brain function and treat them with stem cells and lasers that researchers say could return them to life.
“This represents the first trial of its kind and another step towards the eventual reversal of death in our lifetime,” said Ira Pastor, the chief executive of Philadelphia-based Bioquark Inc.
The start-up was given the go-ahead for the Phase 1 trial by the US National Institutes of Health, but the study will go ahead at the Anupam Hospital in Rudrapur, in northern India, with support from prominent local researchers.
“We just received approval for our first 20 subjects and we hope to start recruiting patients immediately from this first site – we are working with the hospital now to identify families where there may be a religious or medical barrier to organ donation,” Pastor said.
The selected subjects have lost function of their brain stem, meaning they can no longer breathe or attain consciousness, and have to be kept alive artificially, although they maintain a range of automatic bodily functions, which allows them to survive, and even grow.
Four treatments will be administered by Bioquark: injections of stem cells and peptides through a pump to encourage the brain stem to begin growing new working cells, and laser therapy and nerve stimulation, to attempt to increase the level of alertness among the patients.

"To undertake such a complex initiative, we are combining biologic regenerative medicine tools with other existing medical devices typically used for stimulation of the central nervous system, in patients with other severe disorders of consciousness,” Pastor said. “We hope to see results within the first two to three months."
The trial is expected to run for a year.
All four treatments involved have been of legitimate interest to mainstream neuroscientists in recent years, though have yielded mixed results; combining them is a long shot. But Pastor is an evangelist, who believes that medical science’s current views on lifespan and “irreversible” injuries betray a limited outlook.
“In nature, we see evidence of life extension all around us, from species who can turn back biological time via cellular, tissue reprogramming and start life over again like certain hydrozoa, to those who display negligible senescence and age very slowly (certain plants and trees) potentially for thousands of years,” said Pastor, whose degree is in pharmacy, and who has spent most of his career in the commercial departments of pharmaceutical companies.
But for all the lofty goals, any positive result would be an achievement for this early trial.
“It is a long term vision of ours that a full recovery in such patients is a possibility, although that is not the focus of this first study – but it is a bridge to that eventuality,” Pastor said.
And even if brain death reversal is not on the cards just yet, Bioquark hopes that the experiment will provide a pathway to treating the growing number of age-related neurological disorders.
“Through our study, we will gain unique insights into the state of human brain death, which will have important connections to future therapeutic development for other severe disorders of consciousness, such as coma, and the vegetative and minimally conscious states, as well as a range of degenerative CNS conditions, including Alzheimer's and Parkinson's disease,” said Sergei Paylian, the chief science officer of Bioquark.